Table of Contents
What is a mixture?
A mixture is a combination of two or more than two elements that are physically combined together. A mixture is not chemically combined together. A mixture can be of two types; homogeneous and heterogeneous mixture.
Properties of mixture
Some of the properties of the mixture are as follows:
- The substances in a mixture are not chemically combined but are physically combined.
- The separation of the substances can be done easily in a mixture.
- All three states of matter can be combined to form a mixture.
- Mixtures are of two types; homogeneous and heterogeneous mixture.
- There is no change in energy during the making of the mixture.
Types of mixtures
There are mainly two types of mixtures, homogeneous and heterogeneous mixtures. Let us study how these mixtures differ from each other.

Homogeneous mixtures
A homogeneous mixture is a mixture in which the substances dissolved are distributed uniformly and the composition is uniform too. In simple words, we can say that a homogeneous mixture is dissolved completely.

Properties of homogeneous mixtures:
- It has the same uniform appearance.
- The particles in this mixture are very small and cannot be seen easily.
- The mixture is dissolved completely.
- Only one state of matter is observed in this mixture at a time.
- Salt and water solution is an example of this mixture..

Examples of homogeneous mixtures:
The following are some of the examples of a homogeneous mixture.
Solid examples:
- Cement is a mixture of sand, gravel, and water.
- Wood
- Steel
- Alloys
- Plastics
Liquid examples:
- Water
- Blood plasma
- Wines and liquors
- Sugar water
- Mouthwash
- Dishwashing detergent
Gaseous examples:
- Air
- Heliox
- Trimix
- Nitrous oxide
Heterogeneous mixtures
A heterogeneous mixture is the one in which the substances are not distributed uniformly and the composition is also not uniform which means you can easily differentiate between the substances.
Properties of heterogeneous mixtures
- It does not have a uniform appearance.
- The particles in the mixture are easily visible.
- The mixture is not dissolved and hence it cannot be called a solution.
- Different phases of matter can be seen in this mixture.
- Cereals in milk is an example of a heterogeneous mixture.
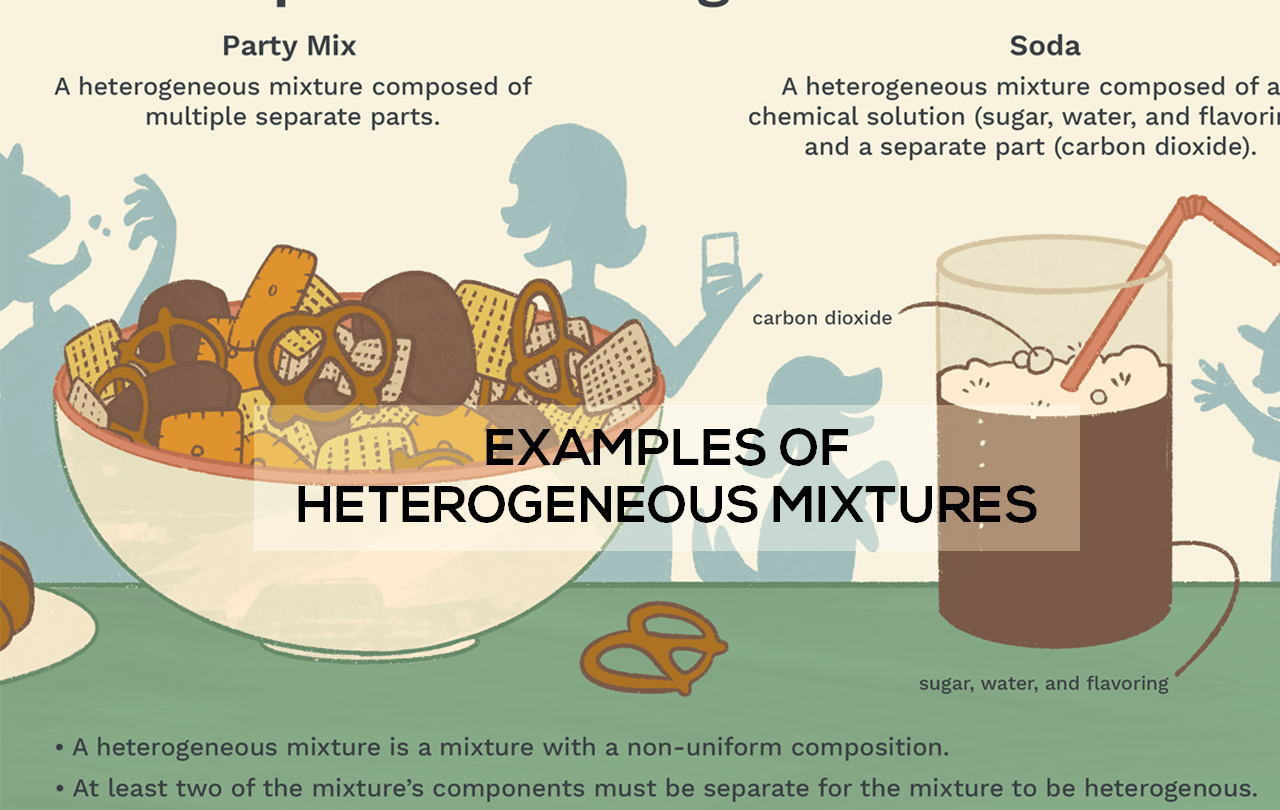
Examples of heterogeneous mixtures:
These are the examples of heterogeneous examples.
Solid examples:
- Egg fried rice
- Pizza with toppings
- Rocks in sand
- Custard with fruits and jellies
Liquid examples:
- Vinegar and oil
- Sand in water
- Cereals in milk
- Vegetable soup
- Baking soda and vinegar
Gaseous examples:
- Mist
- Fog
Solutions
A solution is a type of homogeneous mixture that is formed by combining two or more than two substances. In a solution, one or more than one solute is dissolved in a solvent. A solute in a solution is present in the lesser amount whereas the solvent is present in the greater amount. A solute or a solvent both can be solid, liquid, or gas. A solution is homogeneous and stable.
Solute
The substance in a solution that gets dissolved in the solvent is known as the solute. The boiling point of the solute is higher than that of the solvent. It may be a solid, gas, or liquid and is always present in a lesser amount than the solvent. In an example of a solution that is the seawater, the salt is the solute and the sea is the solvent. In the same way, sugar is an example of solute in the sugar and water solution.
Solvent
The substance in a solution that dissolves the solute in itself is known as the solvent. The solvent is mostly found in the liquid state but can be gaseous as well. The boiling point of the solvent is lesser than that of solute. It is present in a greater amount than the solute in a solution. Water is the most common type of solvent that dissolves all the three states of matter in it easily, and for this reason, it is also known as the universal solvent. The solvent gets evaporated easily.
These are some of the examples of solutions.
- Seawater is an example of a solution in which salt is the solute and water is the solvent.
- In carbon dioxide in water, carbon dioxide is the solute and the water is the solvent.
- In the example of air, nitrogen is the solvent whereas the oxygen, argon, carbon dioxide, and the other gases are the solute.
FAQs
[wps_faq style=”classic” question=”Q: What are the two main types of mixtures?”]A: The two main types of mixtures are the homogeneous and the heterogeneous mixtures.[/wps_faq][wps_faq style=”classic” question=”Q: Is gold a mixture?”]A: No, gold is a pure element and that is why it is not a mixture.[/wps_faq][wps_faq style=”classic” question=”Q: What are the 5 examples of homogeneous mixtures?”]A; Air, seawater, coffee, blood, and water the examples.[/wps_faq][wps_faq style=”classic” question=”Q: What are the two elements of a solution?”]A: Solute and solvent are the two main elements of the solution.[/wps_faq][wps_faq style=”classic” question=”Q: Is solute present in a lesser amount or greater amount in a solution?”]A: Solute is present in a lesser amount in a solution than the solvent.[/wps_faq][wps_faq style=”classic” question=”Q: What are the two main types of solutions?”]A: The two main types of solutions are the aqueous and the non-aqueous solution.[/wps_faq]
Conclusion
In this article, we studied everything about mixtures and solutions. A mixture is basically a combination in which two or more substances are combined. A solution is mainly of two types that are homogeneous mixtures and heterogeneous mixtures.
A solution is a homogeneous mixture of solute and a solvent. A solute is a substance that gets dissolved in a solvent whereas the solvent is the substance that dissolves a solute in itself.

